It has been a great unit to dive into determining the types of bonds, drawing the Lewis dots structure, VSEPR model and analyzing the molecular shape and the polarity.
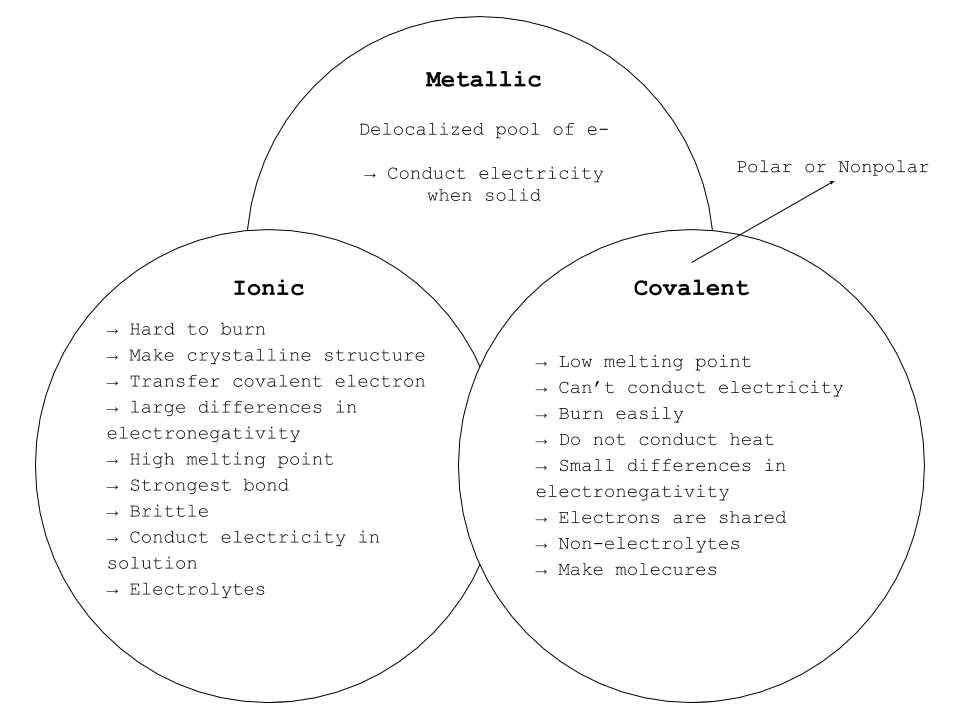
There are three types of bonding such as: Ionic Bond, Metallic Bond and Covalent Bond. In order to identify which elements when combined is an ionic, metallic or covalent bond, these following characteristics will help to determine:
Also we took a lesson on learning about the Lewis Dot Structure, it’s a structure that people would draw to help others visualize the combine elements more closely. It also help us to determined which bond is it through doing the Lewis Dot Structure.
Through drawing the Lewis dot structure it has helped us to better determining the molecular shape and its polarity. As by seeing from the above picture, those are a kind of some diagrams in the class.
Valence-Shell Electron-Pair Repulsion, VSEPR Model is another kind of diagram that people draw. Through this model we can also calculate the formal charge: a valence electrons in the free atoms: non-bonding electrons, bonding e-/2.
Polarity and Molecular Shape:
- Linear: nonpolar
- Trigonal Planar: nonpolar
- Tetrahedral: nonpolar
- Trigonal pyramidal: polar
- Bent: polar
- Trigonal bipyramidal: polar
- Octahedral: nonpolar
Overall, through learning this unit, I’ve learned how to determine the boding and analyzing the molecular shape and more, I’m looking forward to learn more things about chemistry in the next coming unit.